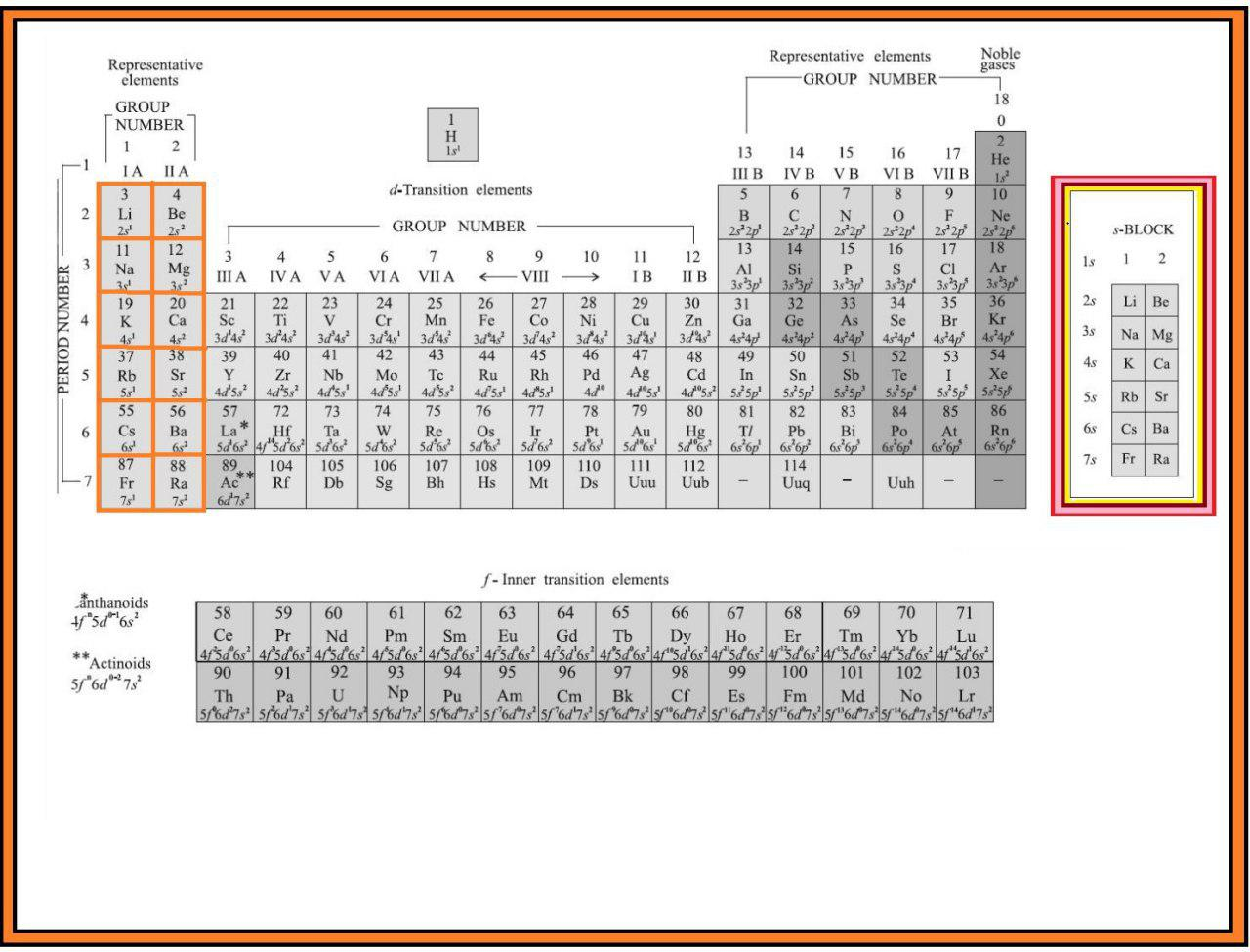
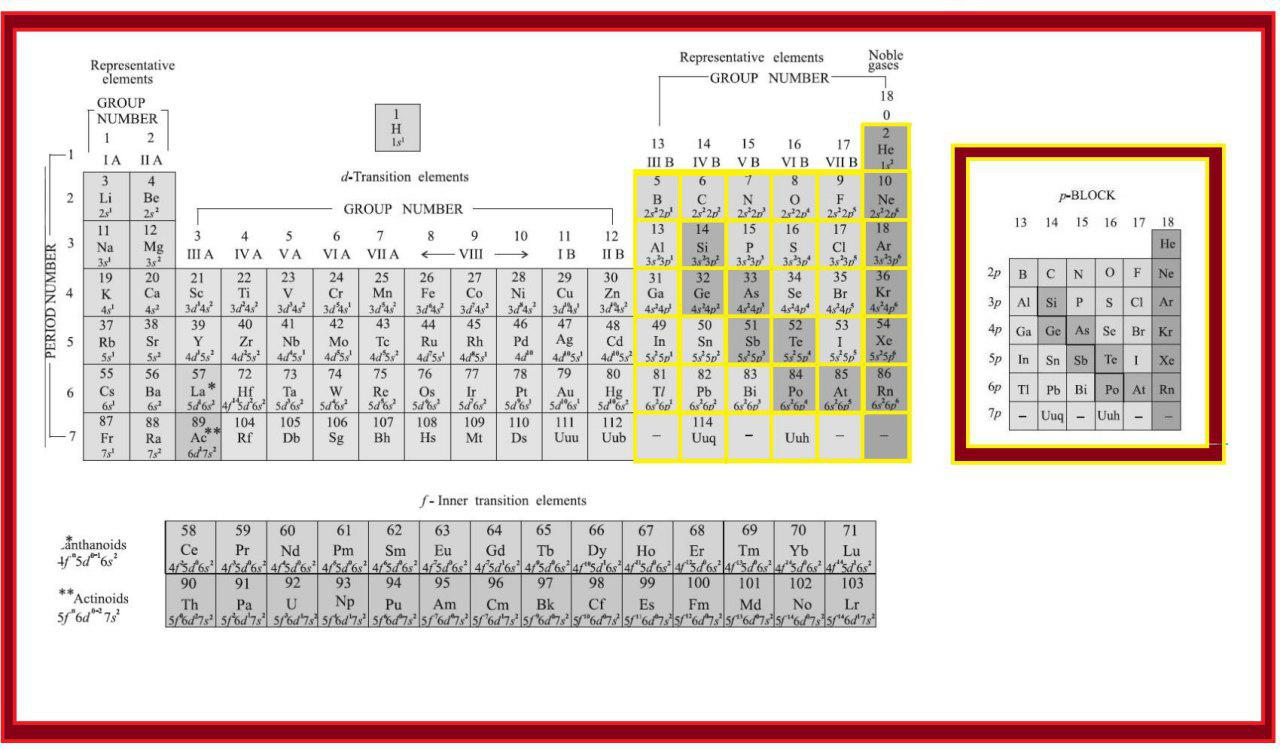
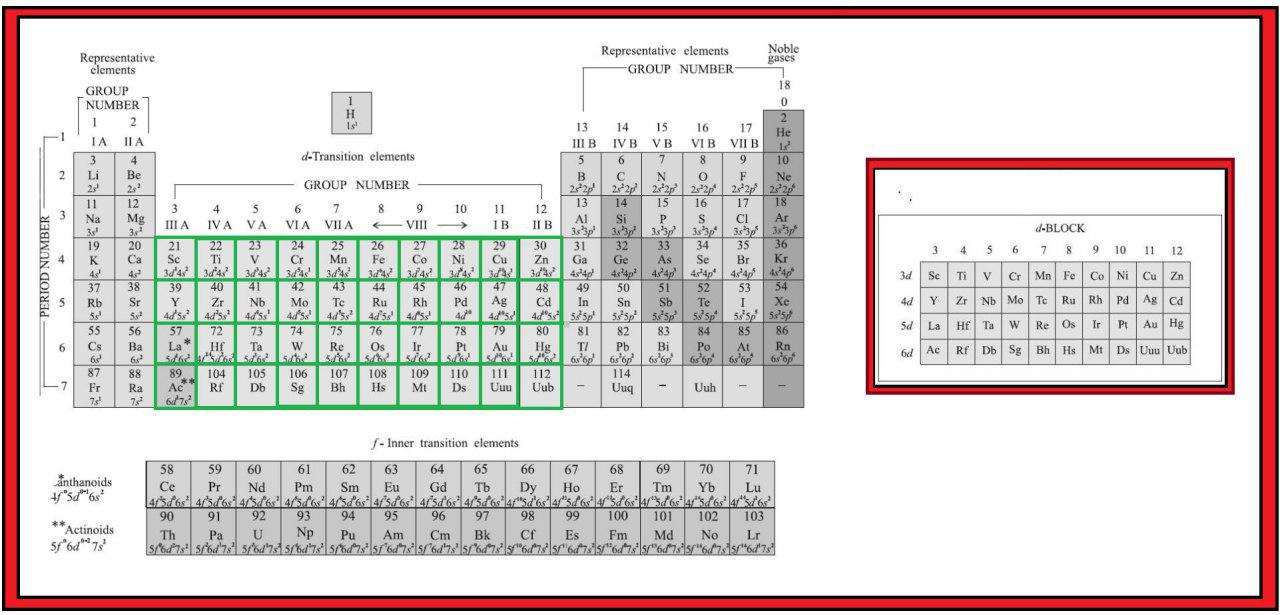
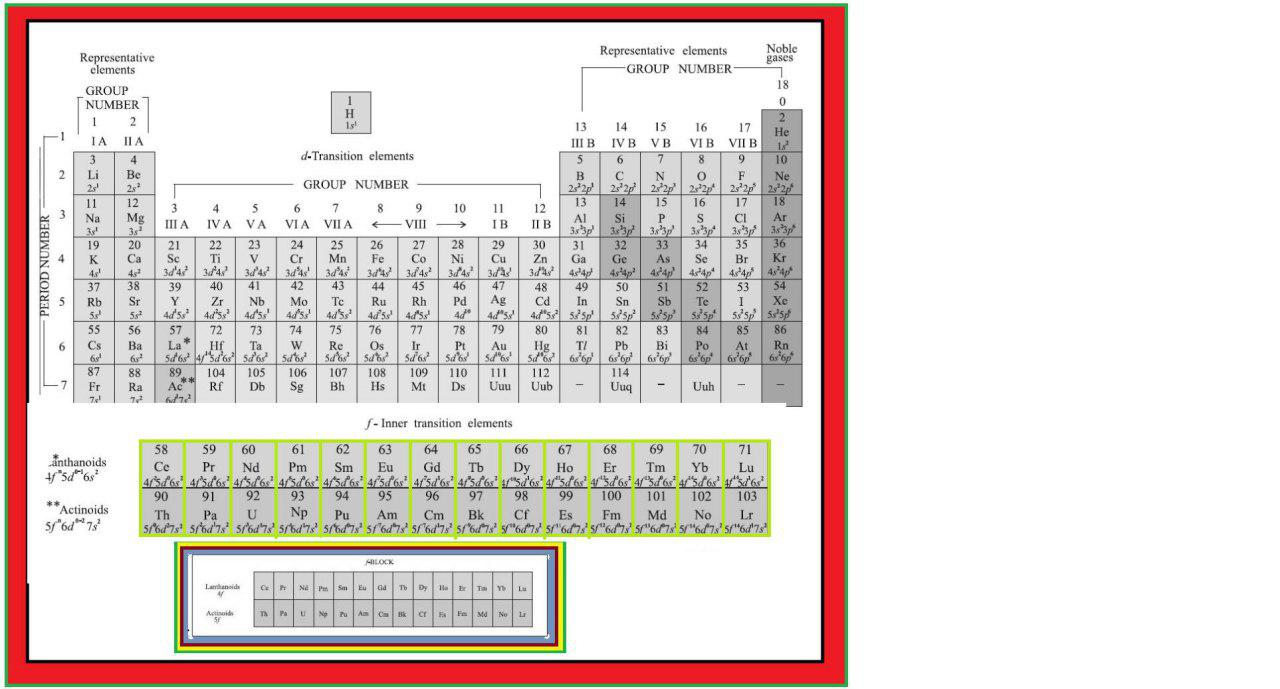
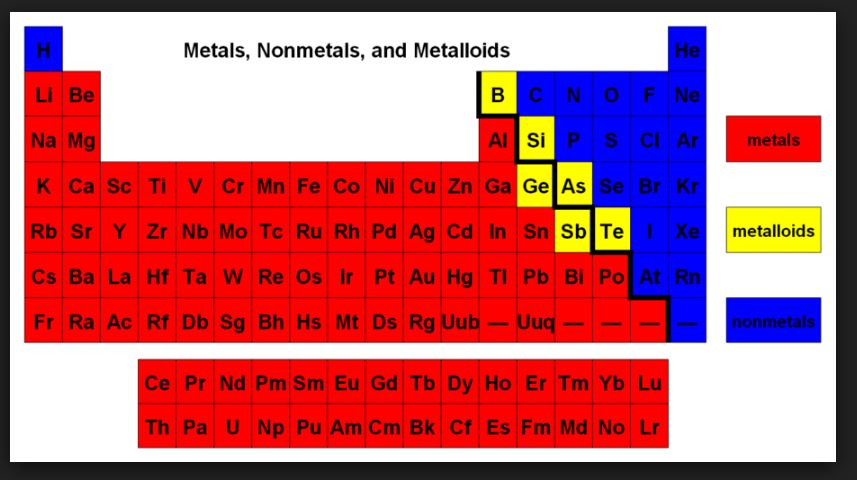
In addition to displaying the classification of elements into s-, p-, d-, and f-blocks, Fig. 3.3 shows another broad classification of elements based on their properties.
`=>` The elements can be divided into Metals and Non-Metals.
`=>` Metals comprise more than `78%` of all known elements and appear on the left side of the Periodic Table.
● Metals are usually solids at room temperature [mercury is an exception; gallium and caesium also have very low melting points (`303K` and `302K`, respectively)].
● Metals usually have high melting and boiling points.
● They are good conductors of heat and electricity.
● They are malleable (can be flattened into thin sheets by hammering) and ductile (can be drawn into wires).
`=>` Non-metals are located at the top right hand side of the Periodic Table.
● In fact, in a horizontal row, the property of elements change from metallic on the left to non-metallic on the right.
● Non-metals are usually solids or gases at room temperature with low melting and boiling points (boron and carbon are exceptions).
● They are poor conductors of heat and electricity.
● Most nonmetallic solids are brittle and are neither malleable nor ductile.
● The elements become more metallic as we go down a group; the nonmetallic character increases as one goes from left to right across the Periodic Table.
● The change from metallic to non-metallic character is not abrupt as shown by the thick zig-zag line in Fig. 3.3.
● The elements (e.g., silicon, germanium, arsenic, antimony and tellurium) bordering this line and running diagonally across the Periodic Table show properties that are characteristic of both metals and nonmetals. These elements are called Semi-metals or Metalloids.
In addition to displaying the classification of elements into s-, p-, d-, and f-blocks, Fig. 3.3 shows another broad classification of elements based on their properties.
`=>` The elements can be divided into Metals and Non-Metals.
`=>` Metals comprise more than `78%` of all known elements and appear on the left side of the Periodic Table.
● Metals are usually solids at room temperature [mercury is an exception; gallium and caesium also have very low melting points (`303K` and `302K`, respectively)].
● Metals usually have high melting and boiling points.
● They are good conductors of heat and electricity.
● They are malleable (can be flattened into thin sheets by hammering) and ductile (can be drawn into wires).
`=>` Non-metals are located at the top right hand side of the Periodic Table.
● In fact, in a horizontal row, the property of elements change from metallic on the left to non-metallic on the right.
● Non-metals are usually solids or gases at room temperature with low melting and boiling points (boron and carbon are exceptions).
● They are poor conductors of heat and electricity.
● Most nonmetallic solids are brittle and are neither malleable nor ductile.
● The elements become more metallic as we go down a group; the nonmetallic character increases as one goes from left to right across the Periodic Table.
● The change from metallic to non-metallic character is not abrupt as shown by the thick zig-zag line in Fig. 3.3.
● The elements (e.g., silicon, germanium, arsenic, antimony and tellurium) bordering this line and running diagonally across the Periodic Table show properties that are characteristic of both metals and nonmetals. These elements are called Semi-metals or Metalloids.